 University of California San FranciscoAbout UCSFSearch UCSFUCSF Health
University of California San FranciscoAbout UCSFSearch UCSFUCSF Health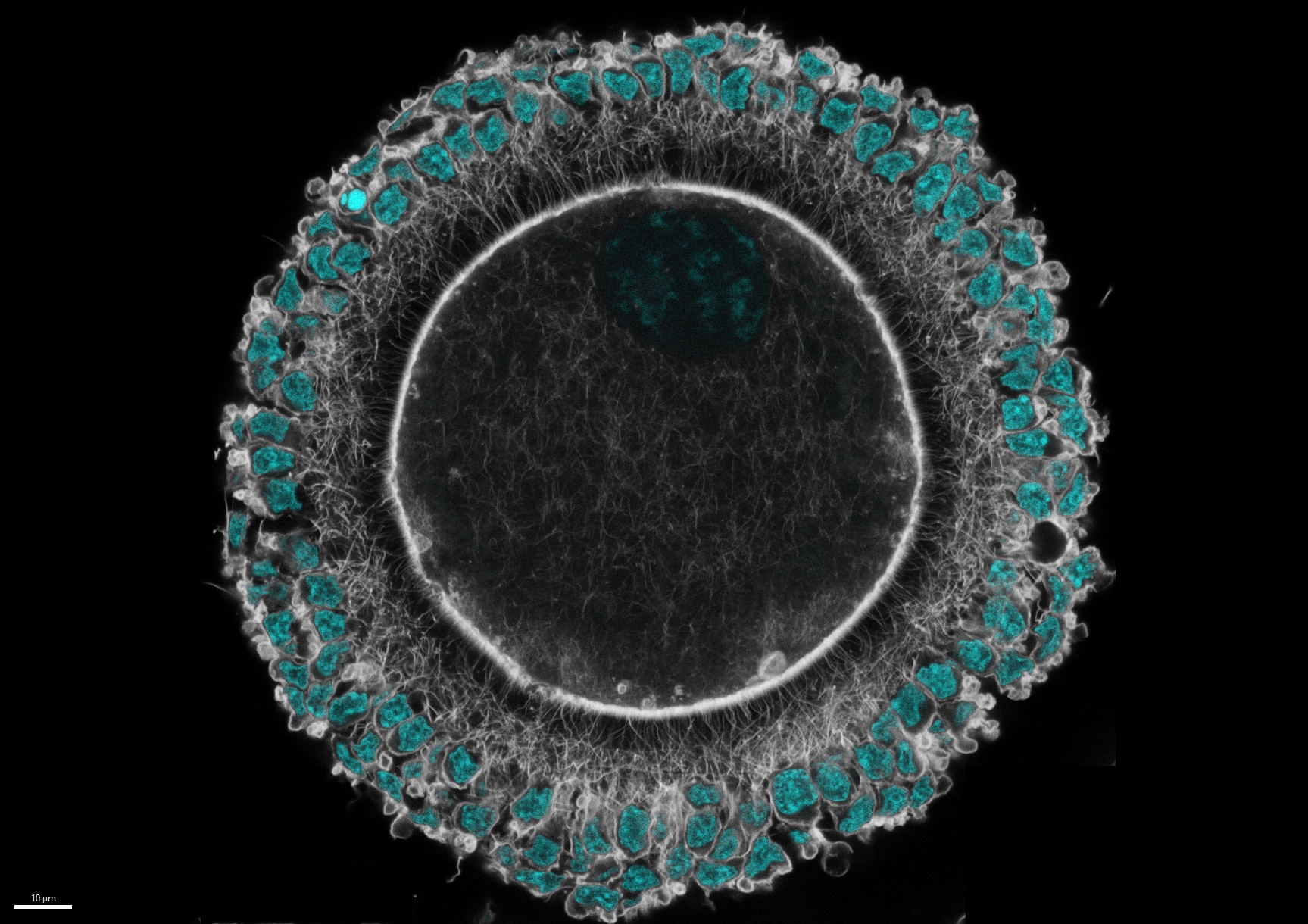

A small number of primordial germ cells established during the first week of mouse development supply all of the eggs or sperm for the reproductive lifespan. This process appears to be surprisingly inefficient, with germ cells lost at multiple steps: some fail to migrate through the embryo, some proliferate less, and many are eliminated by programmed cell death. We are studying the determinants of germ cell fate during development. We use lineage-tracing together with single cell analysis to understand the clonal dynamics of primordial germ cells as they relate to behaviors such as migration, proliferation and apoptosis. At left, germ cells in the mouse fetal testes during scheduled apoptosis (pink cells) are clonally labeled (red, orange and cyan). Understanding the basis of success or failure of nascent germ cells and the relationship to quality of the adult gamete (egg or sperm) has potential implications for the origin of birth defects, infertility, and for the increasingly realistic prospect of growing gametes in a dish.

A woman’s reproductive lifespan is determined by the number of non-growing eggs in her ovary as well as the rate of loss during growth. A critical social and health issue for all people with ovaries is predicting the years of remaining fertility, but current practices measure only mature rather than the most critical reserve population. Further, a link between ovary function and systemic health is suggested by the increased risk for many diseases of aging after menopause. To understand aging of the ovary and how to prolong its lifespan, we are studying the establishment of the ovarian reserve during fetal life and its regulation during adulthood. We are using mouse genetics as well as a rodent model of extreme aging, the naked mole rat. Non-growing and early growing oocytes in a newborn mouse ovary are shown in red.

Distinct from changes to the genetic code, epigenetic mechanisms are important for coordinating rapid cell fate changes, particularly in periods of transcriptional quiescence (such as in early PGCs) or when cell division is not an option (such as in the oocyte). As germ cell development also involves resetting of epigenetic marks as a way to wipe the slate clean for the next generation, interference with this process can potentially wield effects across generations. A current project in the lab concerns the effects of common chemicals and prenatal stress on developing germ cells and their ‘memories’ of exposures.

We develop tools for the study of germ cells in the embryo and postnatal gonad. We established a method to identify and count primordial and growing follicles in the intact mouse ovary by 3D imaging (Faire et al, Dev Biol 2015). We recently extended this approach to the mouse uterus to study the process of embryo implantation.This revealed that the establishment of pregnancy coincides with dynamic changes in the topology of the uterine lining and gland organization (Arora et al, Development 2016). At left, the structure of the uterine glands (multiple colors) around the implanting embryo (red ring) is shown at 4 days of mouse pregnancy.